<NBS5114> BODIPY 665/676 脂質(zhì)過氧化熒光探針
貨號(hào):NBS5114-5mg
品牌:NoninBio
BODIPY 665/676 脂質(zhì)過氧化熒光探針
產(chǎn)品編號(hào) | 產(chǎn)品名稱 | 包裝規(guī)格 | 價(jià)格 |
NBS5114-5mg | BODIPY 665/676 脂質(zhì)過氧化熒光探針 | 5mg | 3593 |
產(chǎn)品簡(jiǎn)介:
BODIPY 665/676是一種親脂性熒光探針,高親和力結(jié)合游離脂肪酸和甘油三酯,具有長波長的最大吸收波長(λex=665nm)和最大熒光發(fā)射波長(Em=676nm)。與常用的脂質(zhì)過氧化探針C11 BODIPY 581/591(NBS5113-1mg)相似,BODIPY 665/676容易被過氧化自由基氧化,一旦氧化,最大發(fā)射波長遷移到605nm(λex =580 nm)。BODIPY 665/676最重要的優(yōu)勢(shì)在于發(fā)射波長是676nm,與絕大部分的熒光素之間無交叉,從而能避免比如自熒光的干擾。BODIPY 665/676具熱穩(wěn)定和高度疏水性特征,從而不會(huì)擴(kuò)散出脂滴。激發(fā)器642nm與最大吸收波長665nm接近,由此,在很低的探針濃度下就能得到強(qiáng)熒光信號(hào)。
產(chǎn)品特征:
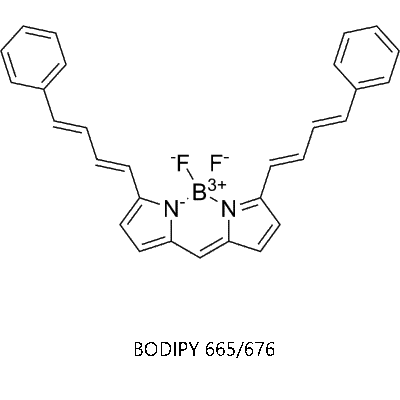
1)同義名:(E,E)-3,5-bis-(4-phenyl-1,3-butadienyl)-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
2)分子量:448.32
3)外觀:藍(lán)色固體
4)激發(fā)波長:665nm
5)發(fā)射波長:676nm
6)化學(xué)結(jié)構(gòu)圖:
保存條件:
-20oC避光干燥保存,收到貨至少12個(gè)月有效。
產(chǎn)品使用:
1.染色工作液的準(zhǔn)備
儲(chǔ)存液的制備:將低溫保存的BODIPY 665/676干粉從冰箱取出后置于室溫至少20min,低速離心后,加入高質(zhì)量DMSO配制成適宜濃度的儲(chǔ)存液,比如:10mM儲(chǔ)存液(即:往5mg BODIPY 665/676加入1.1153ml DMSO,充分溶解后混勻即可),根據(jù)單次用量分裝,≤-20℃保存,盡量避免反復(fù)凍融。
1.2工作液的制備:
BODIPY 665/676用于活細(xì)胞染色的建議工作濃度一般是1-10 μM,請(qǐng)根據(jù)細(xì)胞類型和實(shí)驗(yàn)?zāi)康模瑓㈤單墨I(xiàn),再根據(jù)實(shí)際應(yīng)用摸索最佳工作濃度。用培養(yǎng)基或生理緩沖液稀釋10mM儲(chǔ)存液到所需的工作濃度,現(xiàn)配現(xiàn)用!
2.染色步驟(參考文獻(xiàn))
3.應(yīng)用示例(來自文獻(xiàn),僅做參考)
1) 為了測(cè)定脂質(zhì)過氧化(Lipid Peroxidation),MDA-MB 231細(xì)胞加入BODIPY 665/676(5 μM)孵育30 min。用PBS清洗細(xì)胞兩次以去除多余的染料。用VariosKan酶標(biāo)儀測(cè)定熒光值,結(jié)果以熒光強(qiáng)度(AU)來展示。
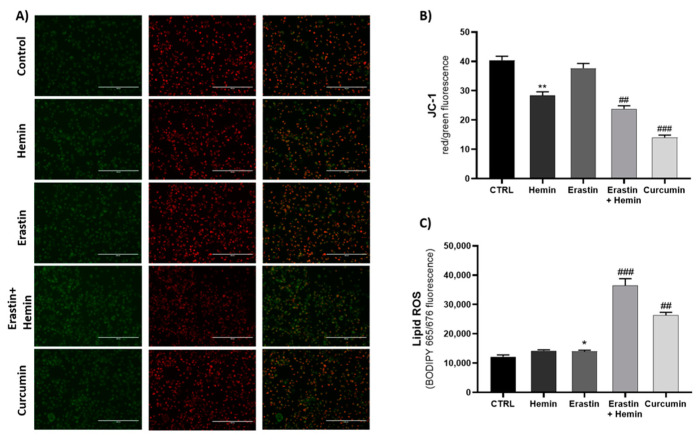
Fig 1. (A) Fluorescence images of JC-1 staining after 8 h of treatment. (B,C) Evaluation of treatment effects on mitochondrial membrane potential and lipid ROS accumulation (* p < 0.05, ** p < 0.005, vs. CTRL; ## p < 0.005, ### p < 0.0005 vs. erastin). The results are expressed as means ± SEM.
2) 為了評(píng)估中性脂滴含量和脂質(zhì)過氧化,細(xì)胞用BODIPY 665/676(1μg/ml)于37℃孵育30 min。孵育結(jié)束后,細(xì)胞用FACSCalibur流式細(xì)胞儀分析。
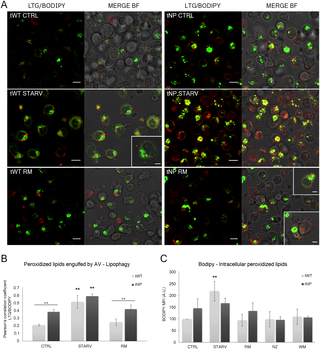
Fig 2. Fig 6. Evaluation of intracellular lipid content and lipophagy by BODIPY? 665/676 (BP).
(A) Single confocal optical sections (~0.8 μm thickness) showing overlay of LTG (green) and BP (red) and the relative merge with bright field (BF), from control, nutrient deprivation and rapamycin- treated tWT and tNP cells. Insets show lipid droplets bound to the outer cell surface, probably newly extruded from the cell, or just below the outer cell membrane, most likely about to be exocytosed by the cell. Bars: 10 μm; 5 μm for insets. (B) Pearson's colocalization coefficient (PCC) for LTG and BP in control and pathologic cells for control, nutrient deprivation and rapamycin administration. Pearson's coefficients were derived from three completely independent experiments with >10 fields per experiment contributing to the cumulative result. Each value is expressed as PCC ± SD; **P < 0.01 vs respective control. The difference between cell lines was determined to be significant by two-way ANOVA (***P < 0.001). Two-way ANOVA with Bonferroni post test revealed a P value < 0.01 (++P < 0.01) between tWT and tNP basal condition and rapamycin-treated cells. (C) Statistical histogram of MFI variation of intracellular BP in tWT and tNP cells for each experimental condition. Mean values were converted to arbitrary units (A.U.) setting control of wild-type cells as 100. Each value is expressed as a relative mean ± SD (Results from n ≥ 3 independent experiments); **P < 0.01 vs respective control.
3)為了測(cè)定脂質(zhì)氧化(lipid Peroxides),細(xì)胞用總內(nèi)皮細(xì)胞處理,之后用BODIPY 665/676(2μg/ml)于37℃孵育30 min。之后上流式,用FlowJo 10.0.0軟件進(jìn)行數(shù)據(jù)分析。
注意事項(xiàng):
1. 整個(gè)染色過程中需注意避光。
2. 為了您的安全和健康,請(qǐng)穿實(shí)驗(yàn)服并戴一次性手套操作。
相關(guān)產(chǎn)品:
產(chǎn)品編號(hào) | 產(chǎn)品名稱 | 包裝規(guī)格 |
100mg | ||
1mg | ||
5mg | ||
10mg | ||
1mg | ||
5mg | ||
5mg | ||
2mg |



